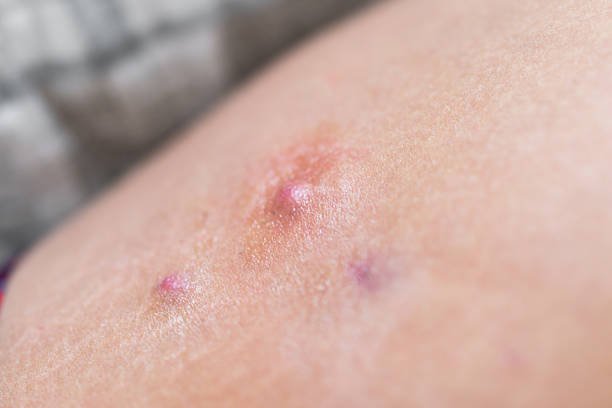
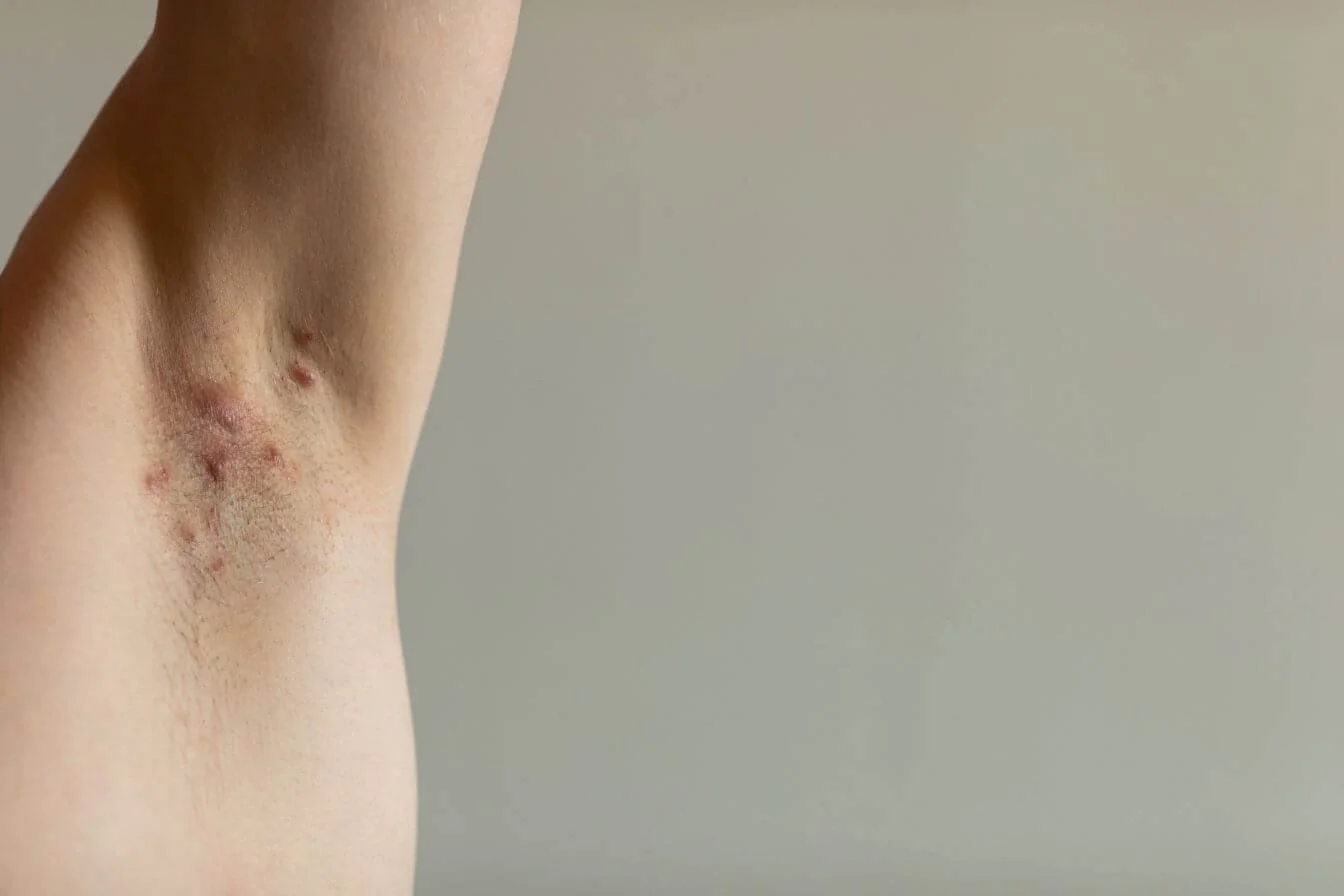
Hidradenitis Suppurativa (HS) Clinical Research Study
Clinical Research Study: Moderate to Severe Hidradenitis Suppurativa
sponsored by Sanofi Aventis Canada Inc.
Interior Dermatology Centre is now enrolling adults for a clinical trial evaluating an investigational medication for moderate to severe Hidradenitis Suppurativa.
Many individuals living with HS experience recurring flare-ups even after antibiotics and standard treatments. This study is evaluating an investigational medication for moderate to severe HS.
Why Participate?
This study includes:
Study medication provided at no cost
Study-related visits, assessments, and procedures provided at no cost
Regular study visits with a dermatologist experienced in HS
Reimbursement for time and travel
An optional extension period may be available after the main study
Your information is confidential, and completing the form does not obligate you to participate.
Who Can Participate?
You may be eligible if you:
Are 18 years or older
Have been diagnosed with moderate to severe HS for at least 6 months
Have HS lesions in two or more body areas
Currently have five or more active lesions
Have previously used antibiotics for HS without adequate improvement
What to Expect
If you qualify and choose to take part:
You will receive subcutaneous injections (abdomen, arm, or thigh)
You will attend regular study visits over approximately 60 weeks
All study procedures are provided at no cost
Reimbursement is provided for time and travel expenses.
Interested in participating?
You can contribute to advancements in dermatology by participating in our clinical trials.
We offer you an opportunity to help discover new treatments and techniques that are advancing the field of dermatology. To submit your interest to participate in our current or future clinical trials, fill out the form and we’ll be in contact to find out if you are eligible to participate in a clinical trial in our Kelowna, BC office.
We’ll let you know if you qualify for any of our current studies or connect with you at a later date if a new study is applicable to your qualifications.
Contact us if you have additional questions info@interiorderm.ca
For more information: https://clinicaltrials.gov/study/NCT07116967
Privacy policy: https://www.interiorderm.ca/privacy-policy