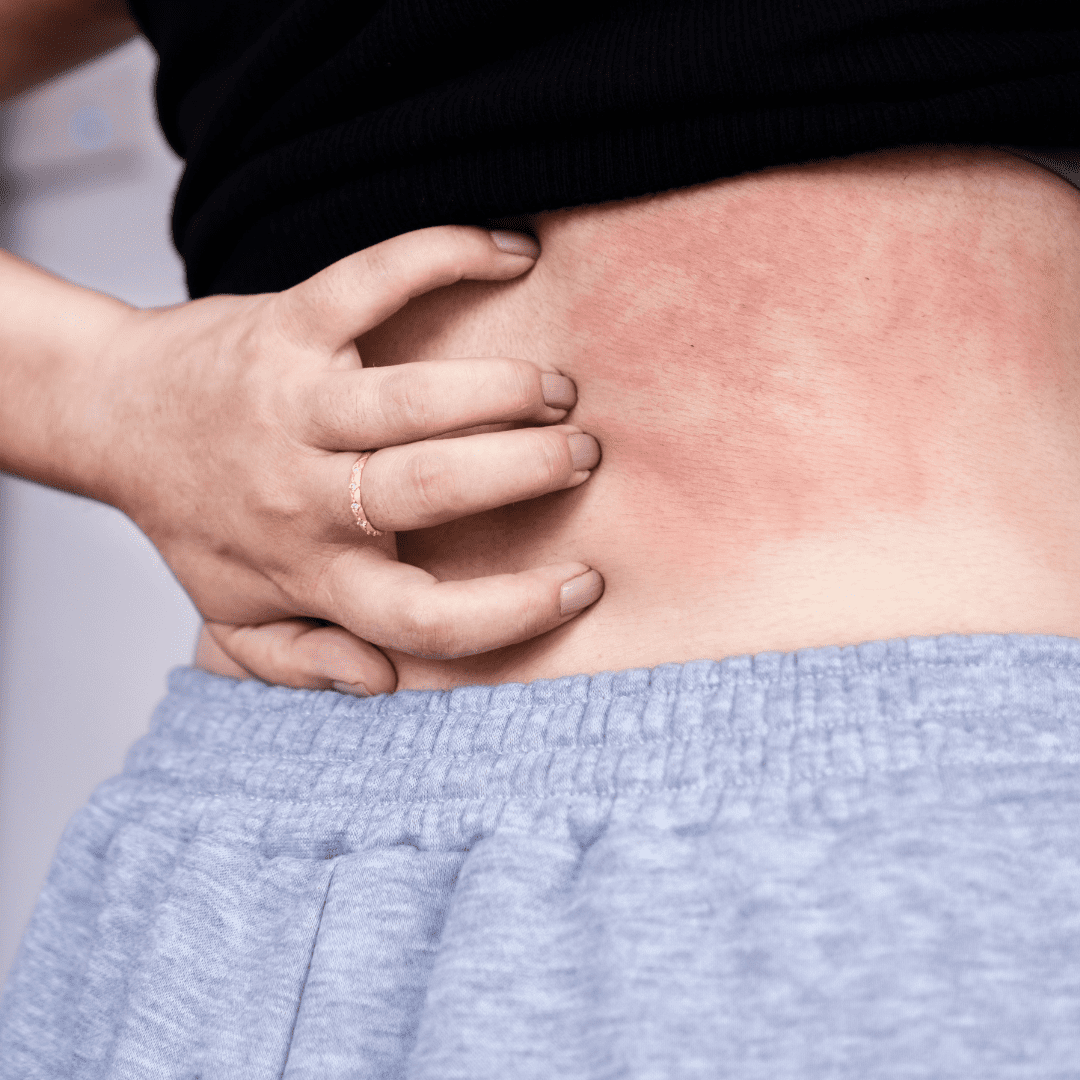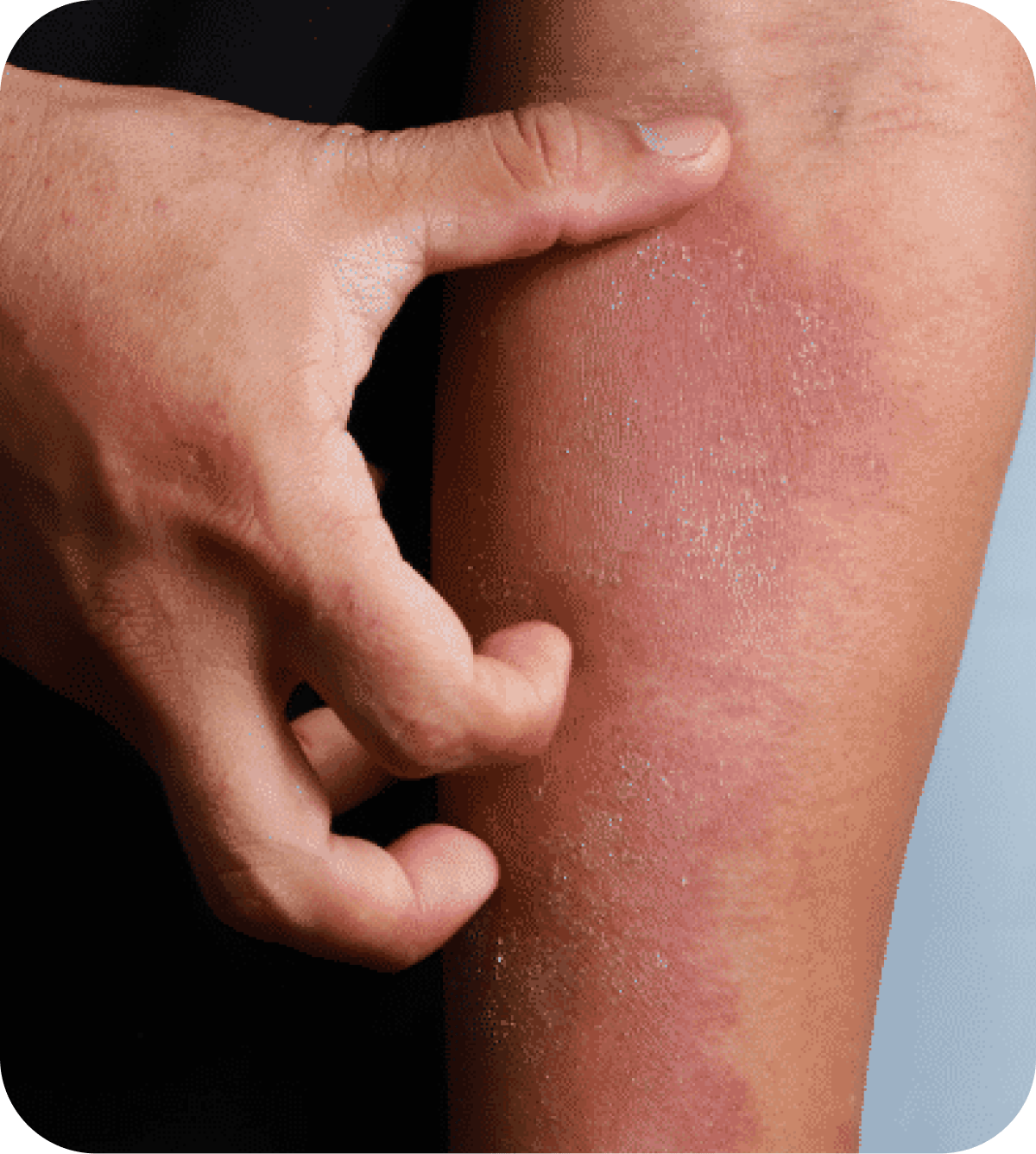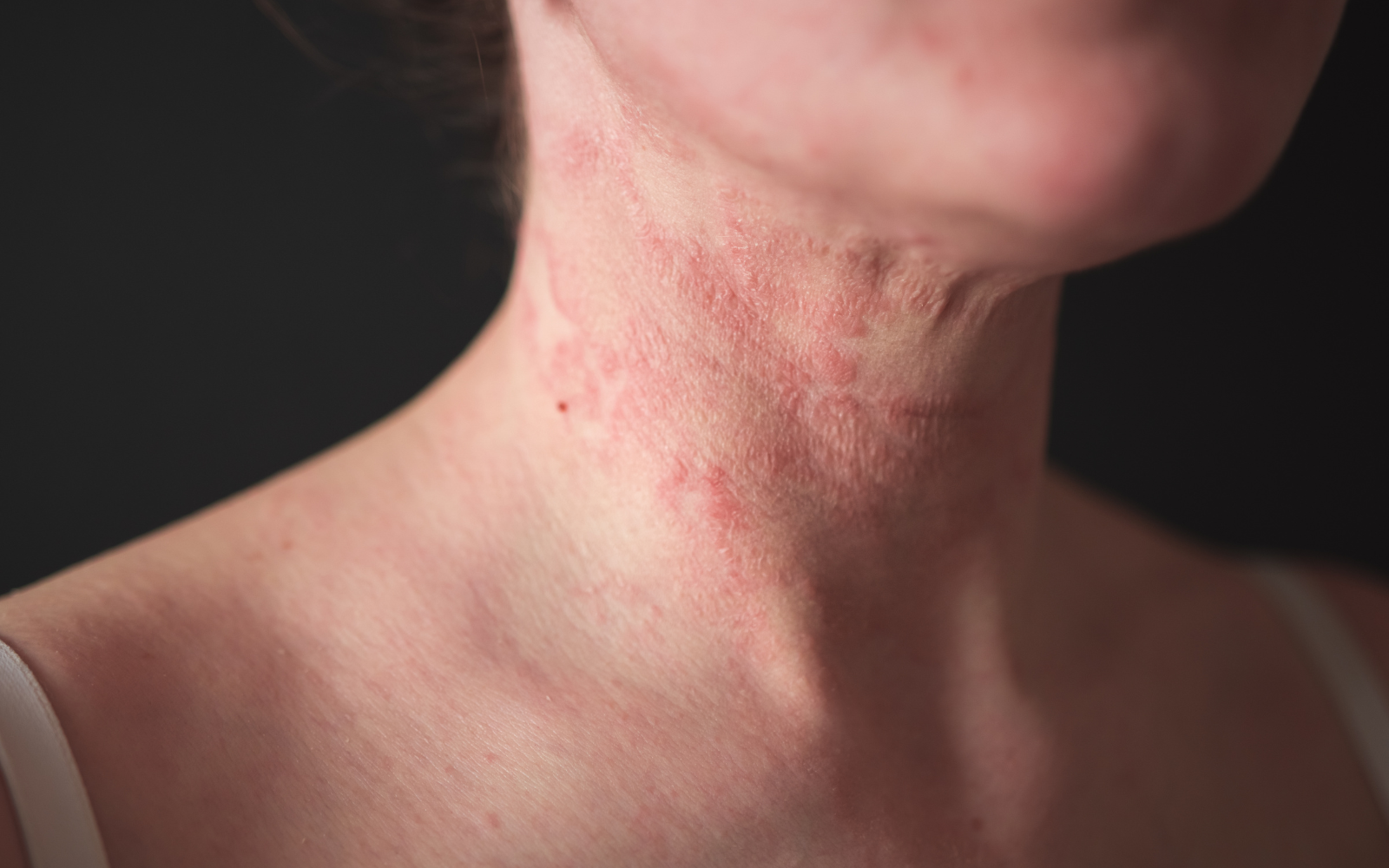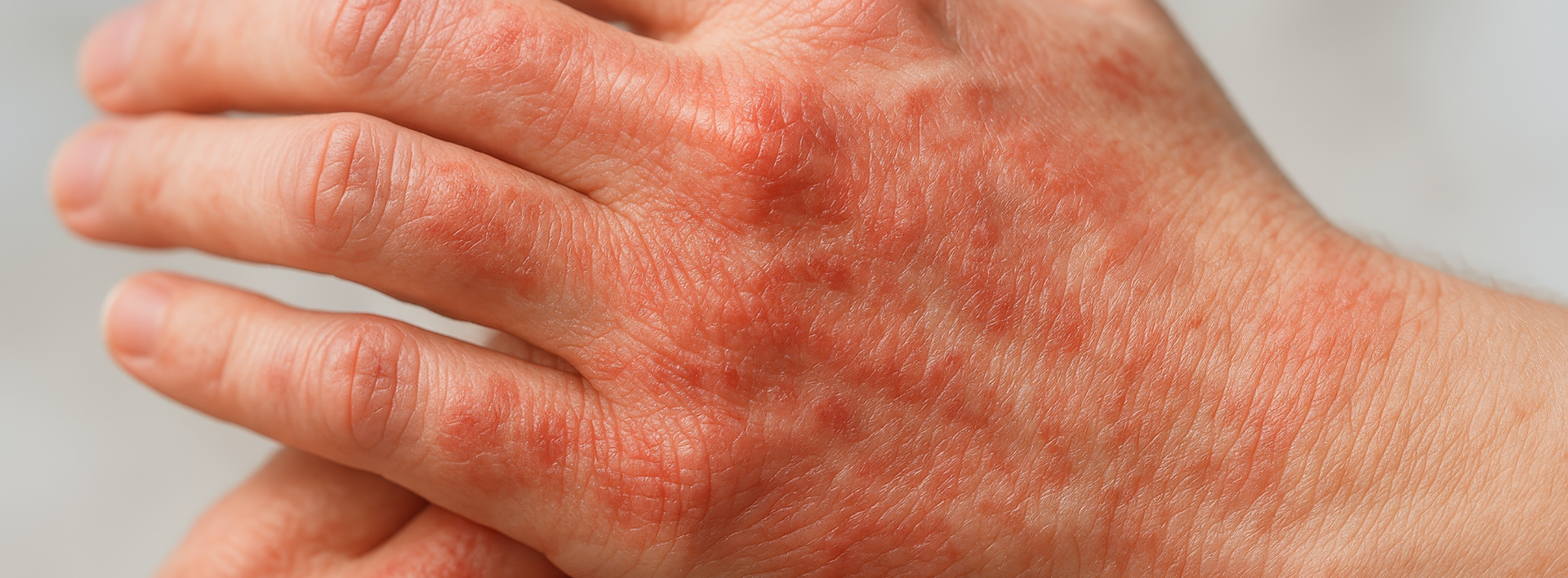
Now Recruiting: Clinical Trial for Moderate to Severe Atopic Dermatitis
Living with moderate to severe atopic dermatitis (eczema) can be frustrating, especially when symptoms keep coming back despite treatment. Interior Dermatology is taking part in a research study looking at an investigational oral medication for chronic atopic dermatitis. We are seeking adults ages 18 to 75 who may qualify to participate.
Who Can Participate?
Adults aged 18 to 75 who have been diagnosed with moderate to severe atopic dermatitis (eczema) may be eligible to participate.
You may qualify if you:
Have had atopic dermatitis for at least 3 years
Have symptoms affecting more than 10% of your skin
(1 palm, not including fingers, is approximately 1% body surface area)
Have tried topical treatments (like creams or ointments) that have not worked well enough
Other eligibility requirements will be discussed with the study doctor.
What to Expect
You will not be charged for participating in the study
You’ll take an oral medication once daily
You’ll meet regularly with a dermatologist (about 24 visits) over 72 weeks
Study related expenses may be reimbursed
How Long is the Study
16 week treatment period (75% chance of receiving active study drug)
52 week open label period where eligible participants will receive the active study drug
Interested in participating?
You can contribute to advancements in dermatology by participating in our clinical trials.
We offer you an opportunity to help discover new treatments and techniques that are advancing the field of dermatology. To submit your interest to participate in our current or future clinical trials, fill out the form and we’ll be in contact to find out if you are eligible to participate in a clinical trial in our Kelowna, BC office.
We’ll let you know if you qualify for any of our current studies or connect with you at a later date if a new study is applicable to your qualifications.
Contact us if you have additional questions info@interiorderm.ca
For more information: https://clinicaltrials.gov/study/NCT07116967
Privacy policy: https://www.interiorderm.ca/privacy-policy